

The Spore Check System is endorsed by the Arizona, California, Ohio and Texas Dental Association. provides sterilizer monitoring services in all 50 states through the Spore Check System. Mesa Labs spore testing is backed by government agencies and independently certified for processing spore test results. However, if you receive a positive spore test, contact your biological monitoring service immediately for assistance. Generally, a single positive spore test probably does not indicate sterilizer malfunction, especially if the process indicators demonstrate sterilizer effectiveness. Also check the sterilizer for any obvious inconsistencies. Ensure that the process indicator has not expired and that testing protocol has been met. Principle When a heat-fixed smear is flooded with aqueous malachite green solution (the primary stain) and steamed, the heat assists the stain in penetrating through the spore. If a process indicator turns positive, then retest with the process indicator immediately. Spore test results should be maintained for at least one year to track any deficiencies.Ĭommon factors for improper sterilization include chamber overload, excessive packaging material, inadequate exposure time, incorrect temperature/pressure settings, failure to preheat sterilizer, interruption of the cycle, and expired chemical solution (chemiclaves only). It is good practice to place the spore test strip in a different location of the sterilizer each week to help identify any “cold spots” within the sterilizer. If there are no instructions, place the spore test strip within a wrapped set of instruments in the most difficult area to be sterilized, which is normally the lower front area of the sterilizer.

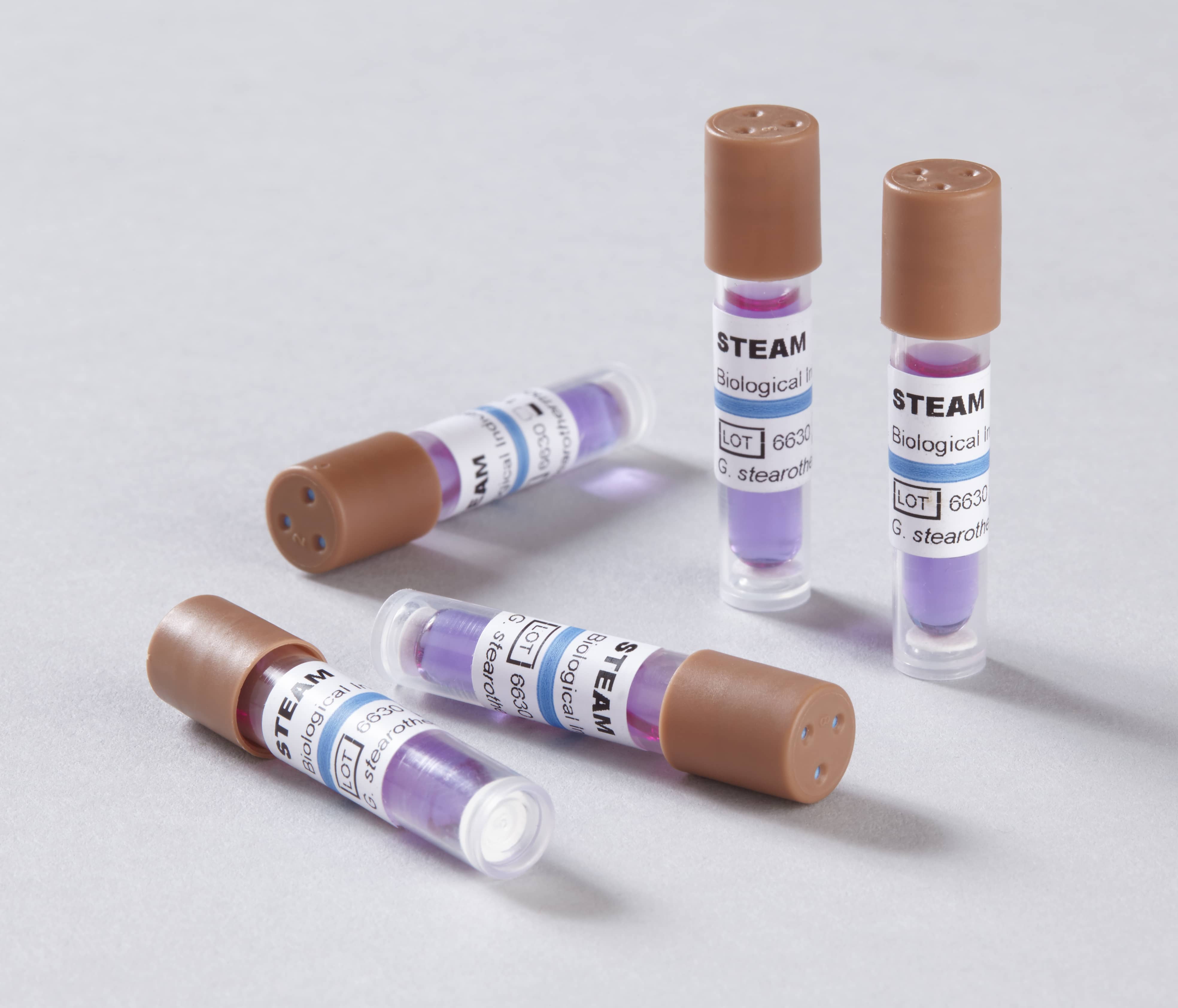
The spore strips should be placed according to the sterilizer manufacturer’s instructions. When spores are killed during a sterilization cycle, it is assumed that all microorganisms have been destroyed and sterilization is effective. Biologic indicators consist of highly resistant, nonpathogenic bacterial spores. spore tests, is considered the standard practice in dentistry, and when properly implemented, is a vital part of infection control verification. Regular sterilizer monitoring using biologic indicators, i.e. Because Spores Dont Lie Spore tests help assess the sterilization and compliance of ridding highly resistant microorganisms. Online Access to your account is available saving paper and time Assurance. Boost Labcare are the only UK provider of Mail-in Spore Testing for Tattoo and piercing studios, dental and cosmetic surgeries. All rights reserved.The Centers for Disease Control and Prevention (CDC) recommends, and most states require, dental offices to monitor their sterilizers weekly using a spore test. Choose to have your spore testing results mailed, faxed or e-mailed. Published by Houghton Mifflin Harcourt Publishing Company. Copyright © 2014 by Houghton Mifflin Harcourt Publishing Company. At Mesa Labs, we streamline the process of testing and tracking results with our user-friendly online dashboard. Learn more about proper placement of spore vials inside your sterilizer. The American Heritage® Student Science Dictionary, Second Edition. What’s Included in Your Testing Service Maintaining sterile tools is paramount to ensuring the safety of your patients and clients while achieving compliance with regulations, such as the ADA or state Dental Boards. In Office Biological Monitoring (Spore Testing) Be sure your sterilizer is functioning properly by performing biological monitoring at least once weekly following CDC recommendations.


 0 kommentar(er)
0 kommentar(er)
